Imperial College calls for volunteers aged 18-55 from London, Bristol and Southampton to test potential coronavirus vaccine – and will pay people up to £625 to take part
- Volunteers asked to come forward to take part in first British human vaccine trial
- Matt Hancock said the vaccine will be the best way to beat virus ‘in the long run’
- Oxford scientists have genetically modified a common cold virus to make the jab
- They will test the vaccine on up to 510 people aged between 18 and 55
- Researchers hope to give people internal protection against COVID-19
- Trials are recruiting volunteers in London, Bristol and Southampton
- To find out about taking part, go to www.covid19vaccinetrial.co.uk/volunteer
- Learn more about how to help people impacted by COVID
A call has been made for volunteers to take part in the first human trials of a new coronavirus vaccine in Britain.
Imperial College London and University Hospital Southampton asked for people to take part in the study to test if a potential inoculation is effective in tackling the disease.
The COVID-19 vaccine developed at the University of Oxford will be trialled on humans in the UK from Thursday this week.
Anyone who is healthy and aged between 18 and 55 can take part at Imperial College London, University Hospital Southampton plus Bristol Children’s Vaccine Centre.
Those who take part in the trial at these centres could be paid up to £190 to £625 reimbursement for their time.
Health Secretary Matt Hancock today said he was ‘throwing everything at’ Britain’s attempt to develop the first vaccine in the world.
The Government will give the scientists in Oxford an extra £20million to help with their trials, Mr Hancock said, and a further £22.5m to a project at Imperial College London.
Despite development of a new vaccine normally being around 18 months, researchers at Oxford believe large-scale production could be under way as early as September – only nine months after the virus came to light in Wuhan, China.
Scroll down for video.

NHS staff carrying out coronavirus tests at a facility in Bracebridge Heath, Lincoln, as the UK continues its lockdown to help curb the spread of the virus

Health Secretary Matt Hancock announced trials would begin this week at today’s daily Government briefing

An incubator full of hyperflasks used in the development of the ChAdOx1 vaccine candidate at the Clinical Biomanufacturing Facility (CBF) in Oxford. Human trial for this vaccine will start in Britain on Thursday

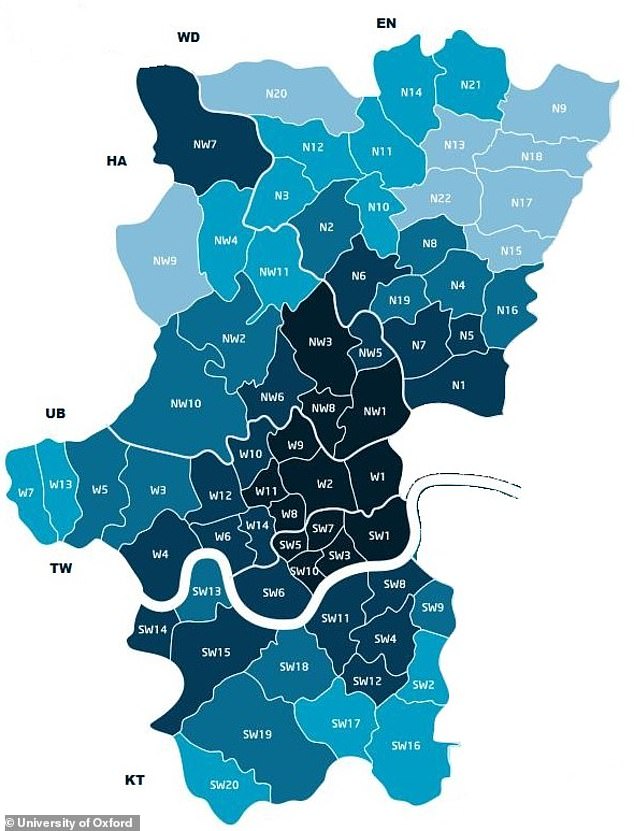
Imperial College London (above its catchment area) has put a call out for volunteers to take part in coronavirus trials, due to start on Thursday
The Oxford vaccine, known as ChAdOx1 nCoV-19 will be trialled on up to 510 people out of a group of 1,112 aged 18 to 55. It is recruiting volunteers in London, Bristol and Southampton. The Oxford Vaccine Centre is taking part but is not currently recruiting volunteers.
It is the first British-made vaccine to go into real-world trials and carries with it huge hopes that it will provide a key to getting out of lockdown and banishing COVID-19.
Imperial Medicine tweeted today: ‘The Imperial College NHS Trust are looking for healthy volunteers to participate in a #COVID19 #vaccine trial, for which they will receive up to £190-£625 reimbursement for time, travel and contribution to the trial.’
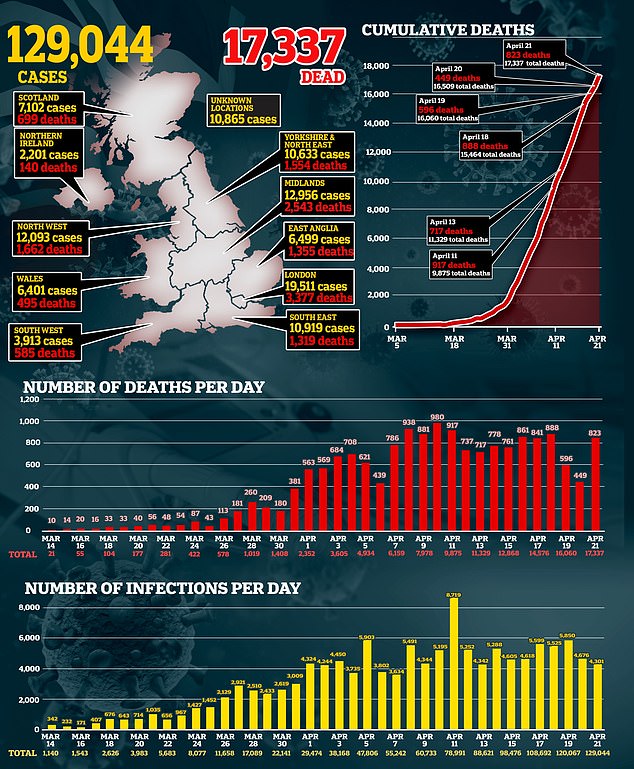
The virus has now infected more than 125,000 people and killed 17,339 in the UK and the UK is on course to end up one of the worst-hit nations in the world.
Mr Hancock said developing vaccines is an ‘uncertain science’ which usually takes years but that manufacturing capacity will be ramped up in case the jab is a success and is suitable to roll out to the public.
The trial will take six months and is limited to a small number of people so scientists can assess whether it is safe and effective without using huge amounts of resources – each patient must return for between four and 11 visits after the jab – and without the risk of large numbers of people being affected if something goes wrong.

The vaccine has been developed in just four of months since the coronavirus outbreak started and will now be distributed to around 500 trial participants
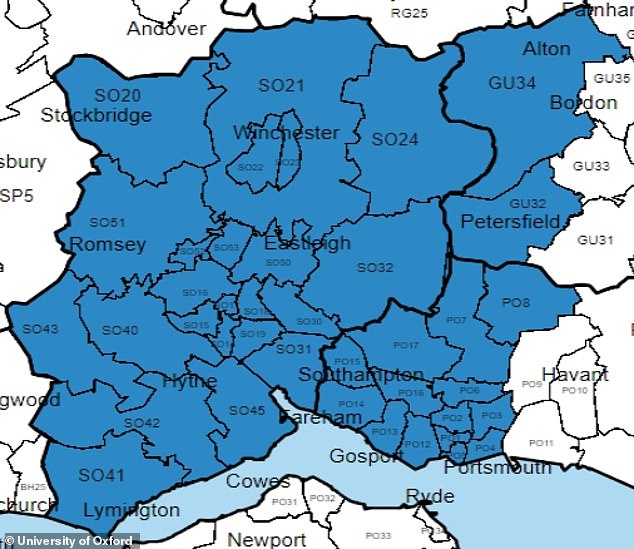
University Hospital Southampton (above its catchment area) is one of the centres recruiting volunteers for the new virus trial


The trial will be taking place at University Southampton Imperial College London, Bristol Children’s Vaccine Centre and University Hospital Southampton (which tweeted above)
Speaking at today’s coronavirus briefing at Downing Street, the Health Secretary said: ‘In the long run the best way to defeat coronavirus is through a vaccine.
‘After all, this is a new disease. This is uncertain science, but I am certain that we will throw everything we’ve got at developing a vaccine.
‘The UK is at the forefront of a global effort. We’ve put more money than any other country into the global search for a vaccine and, for all the efforts around the world, two of the leading vaccine developments are taking place here at home at Oxford and Imperial [College London].
‘Both of these promising projects are making rapid progress and I’ve told the scientists leading them that we’ll do everything in our power to support.’
He pledged a total of £44.5million to the projects in Oxford and London to enable scientists to go ahead with trials and getting the vaccine used in people.
Chief Scientific Adviser Sir Patrick Vallance yesterday warned the success of a new vaccine is a ‘long shot’.
He told the Guardian: ‘All new vaccines that come into development are long shots. Only some end up being successful.
‘Coronavirus will be no different and presents new challenges for vaccine development.
‘This will take time and we should be clear it is not a certainty.’
There are currently more than 100 vaccine projects under way around the world, Sir Patrick said.
The new jab is based on an adenovirus, which is the type that causes common colds, which was taken from chimpanzees and damaged so it is unable make humans ill.
The virus was genetically engineered so that it makes ‘spike’ proteins found on the outside of the COVID-19 viruses and are essential to its ability to infect people.
By injecting these proteins into the body but without the rest of the coronavirus, scientists hope to train the immune system to recognise those proteins as a disease-carrying invader and work out how to attack it.
If successful, this will mean that a vaccinated person will not become ill if they catch the real coronavirus because their body has already learned to attack the proteins that will be on the outside of it. Therefore, the immune system will in theory be able to destroy it before it is able to cause any symptoms.
Mr Hancock said: ‘The team have accelerated that trial process, working with the regulator, the MHRA, who have been absolutely brilliant.
‘And as a result, I can announce that the vaccine from the Oxford project will be trialled in people from this Thursday.
‘In normal times, reaching this stage would take years and I’m very proud of the work taken so far.’
University of Oxford scientists are confident they can get the jab for the incurable virus rolled out for millions to use by autumn.
Whether a vaccine can be developed and distributed around the country before the end of the year will depend on the speed of the trials and whether it can be produced on a large-scale once it has been proved to be successful.
Mr Hancock added: ‘If either of these vaccines safely works, then we can make it available as soon as humanly possible.
‘After all, the upside of being the first country in the world to develop a successful vaccine is so huge that I am throwing everything at it.’

A scientist working on the team developing the ChAdOx1 vaccine candidate against COVID-19 pouring fluids at the Clinical Biomanufacturing Facility (CBF) in Oxford
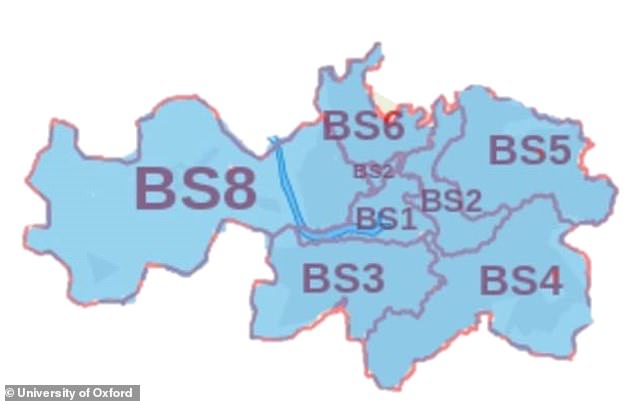
Bristol Children’s Vaccine Centre (pictured its catchment area) is one of the three centres recruiting for volunteers to take part in coronavirus human trials
Clinical nurse Wendy Dickinson receiving a BCG injection in the trial clinic at Sir Charles Gairdner hospital in Perth, Australia, yesterday
Professor Saul Faust, a director of clinical research at University Hospital Southampton, said that if the trials are successful the vaccine could be available for larger trials later this year and, later, for public use.
She said: ‘Vaccines are the most effective way of controlling outbreaks.’
Explaining how the vaccine that will be trialled at the hospital works, Professor Faust added: ‘This vaccine aims to turn the virus’ most potent weapon, its spikes, against it – raising antibodies that stick to them allowing the immune system to lock onto and destroy the virus.’
Around half of the people in the trial will be given the COVID-19 vaccine candidate and the others will receive a ‘control’. For this, researchers will use the MenACWY vaccine, which is a vaccine already used by the NHS to protect against meningitis.
Work on the vaccine, developed by clinical teams at the Oxford University’s Jenner Institute and Oxford Vaccine Group, began in January.
Britain will join only the United States – with two studies – and China in beginning human trials. These trial started in March, putting them around a month ahead of the Oxford study.
Professors Andrew Pollard, Sarah Gilbert and Adrian Hill, who are leading the trial said in a statement: ‘The Oxford Covid vaccine team are delighted with Tuesday’s announcement by the Secretary of State for Health of funding for the evaluation of the new COVID19 vaccine.

A NHS or care worker being tested by a soldier for COVID-19 at a drive-through testing centre in a car park at Chessington World of Adventures yesterday

A police car in a quiet Fitzroy Square in London today. The British government has extended the lockdown restrictions first introduced on March 23
‘This week we will start the process of vaccine evaluation in our first human studies and are currently focusing all efforts on preparing for the start of the trials.
‘Although it seems like a very long time since the work started, in reality it is less than four months since we first heard of an outbreak of severe pneumonia cases, and began to plan a response.
‘Our brilliant team has been working tirelessly to get to this point using our skills and experience in vaccine development and testing, and will do the best job possible in moving quickly whilst at all times prioritising the safety of the trial participants.’
The vaccine is made from a version of a common cold virus combined with genes that make proteins from the COVID-19 virus (SARS-CoV-2) called spike glycoprotein, which play an essential role in the infection pathway of the SARS-CoV-2 virus.
The UK has today announced another 828 deaths from the coronavirus today, taking Britain’s total number of victims to 17,337.
Officials diagnosed 4,301 more people with the virus in the past 24 hours, marking the lowest increase in confirmed cases in a fortnight, since April 7 when 3,634 people were diagnosed.
This rise in fatalities is the biggest increase since Saturday, April 18, (888) and almost double the number that was announced yesterday (449).
Tonight Mr Hancock insisted the virus lockdown must stay until there is no risk of a second peak.
But scientists warned the outbreak might not be fading and have been telling ministers behind the scenes that control of the outbreak is still so uncertain that even slight changes to the curbs on normal life could result in a disastrous resurgence of the disease.
