Chances are, someone you know has been affected by mental health problems. With an estimated world-wide burden of 300 million people, depression can be a severely debilitating condition. In fact, in the past year alone, more than 16 million adults in the US have experienced a major depressive episode.
While many of those who seek help are able to find solace in therapy or medication, treatment-resistant depression is not uncommon. Clinicians like Stanford’s Dr. Nolan Williams have been studying the applications of transcranial magnetic stimulation (or TMS) for a variety of neurological conditions. We sat down with Dr. Williams to discuss his lab’s use of TMS for the treatment of depression and OCD, and the general status of this field.
Mohammad Saleh, Medgadget: Tell us about your background and how you came to work in this field.

Dr. Nolan Williams, Stanford: I am trained as both a psychiatrist and a neurologist. I was trained at the University of South Carolina under Mark George, who is the inventor of using TMS as a therapeutic tool for neuropsychiatric conditions. I completed residencies in research and clinical fellowship under him. I was recruited to Stanford after that, and I have spent the last four years building my Brain Stimulation Lab. I have experience treating people with treatment-resistant depression, treatment-resistant OCD, treatment-resistant Tourette, Parkinson’s disease and others – both using implanted devices and noninvasive neuromodulation devices.
Medgadget: Can you give the readers a bit of an overview of what Transcranial Magnetic Stimulation (TMS) is and how you are using it to treat mental illness?
Williams: TMS is a term used for electromagnetic devices that produce depolarization of cortical neurons. It takes advantage of Faraday’s Law – pulsing a magnetic field can induce a current under electrically conductive substances. Primarily what we are doing is that we are inducing an electrical current with a magnetic field – enabling us to pass through low conductive substances and get it only into the brain. In theory, you could stimulate with direct electrical stimulation. The problem is – to get anywhere near what you can do with TMS, you would burn the skin.
TMS has been around since 1985, in a form that would allow one to affect the brain. It was used primarily as a motor physiology tool, not as a therapeutic tool. Eventually, Mark George in 1995 published the first cases of using repetitive Transcranial Magnetic Stimulation as a way of therapeutically changing brain networks. If you stimulate once, it is a probe – I can put it over the area of your motor cortex responsible for hand movement and I can make your thumb move. But if I put it over the area of the brain that regulates mood or how compulsive or obsessive one is, and repeatedly stimulate, I send a signal into this network to encounter information or react to environmental stimuli in a different way, ultimately changing behavior.
Medgadget: How can you be accurate with this? How do you know that stimulating a particular area related to, for example, compulsivity is not going to make people even more compulsive?
Williams: There are three essential elements to repetitive TMS. There is the diagnosis, the target, and the direction of the stimulation. You want to have a diagnosis, what neuroanatomy you are targeting, and which direction to send the brain into. In some cases, if you send the brain in the wrong direction at the right target, you can make symptoms worse. If you stimulate or inhibit the brain at the right target, for the right diagnosis, it is either going to work completely, partially, or not at all. You rarely see anybody get worse in therapeutic TMS. You can make the brain fire in a way that makes it more likely to be excitable, or you can send an inhibitory pulse sequence and you can make the brain less likely to be excitable.
In the case of all the major TMS applications that the FDA has approved thus far, we know the correct direction (inhibitory vs stimulatory), the correct target (at least for some patients) and the condition linked to those two variables. In the case of depression, the idea is that the left dorsolateral prefrontal cortex, in at least some people, is hypofunctional. Meaning that whatever happened to get the person to that point in their disease has led to decreased functionality in that region. In a simplistic view – all you would need to do is get the magnetic coil over this region and excite it.
The beauty of TMS is that if I need to excite the left dorsolateral prefrontal cortex, I don’t also excite the motor cortex or the visual cortex or anything else. It’s similar for OCD, where we’re going after the anterior cingulate and not really exciting areas that are nearby. So, the stimulation is focal to the brain region you are trying to get to.
Medgadget: So, you’re essentially trying to do what pharmaceutical drugs do, except without any of the side effects because you’re targeting a specific area of the brain instead of flushing it with chemicals?
Williams: I do not know if this is doing what drugs are doing. There are certain aspects of this that certainly seem to mimic some drugs. For instance, the dopamine release that happens after you do TMS at a left dorsolateral prefrontal cortex is similar. But that is not exactly the same thing as having a drug block the reuptake of dopamine. It is probably doing something mechanistically different than what the drug is doing.

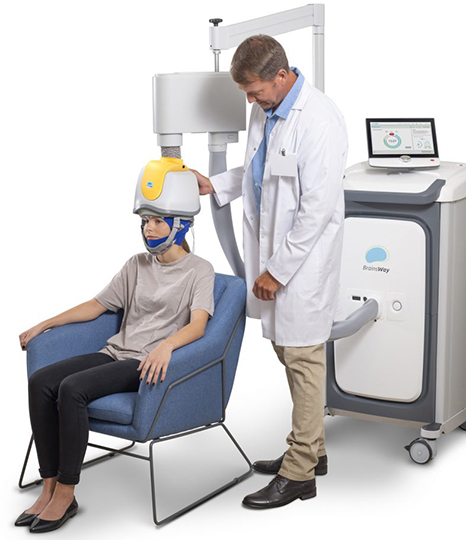
BrainsWay TMS Helmet
The idea is that it is restoring function. The interesting thing is, in the over 500 patients I have treated over the last decade of my life, I have never had a patient come say, “My depression is gone, but I feel this weird side effect.” You hear the patients say, “I feel normal again,” or something like that. That is not necessarily the case with psychiatric medications. People do often report experiencing side effects that make them feel a little bit different than what they would expect their “normal” to be.
Medgadget: Do you have any idea about the statistics related to whether or not there are any side effects of TMS?
Williams: People do post-market surveillance for the FDA. Patients might experience headaches or a minor pain at the site of the treatment. However, these side effects are typically brief and cease after the first few sessions. They’ve also found a small seizure risk of one in 30,000 post-market incidents. But there is a seizure risk with drugs like bupropion as well – it is not unique to TMS. It never lasts more than a minute or ever required long hospitalization, and people usually recover within 15 or 20 minutes. It is very rare for somebody to have TMS-related seizures. That is really it, other than sensory experiences during the stimulation which were related to activating nerves in the scalp. But there are no known persistent side effects of TMS.
Medgadget: Walk us through how complicated the procedure is. Does this need to be specifically done by clinicians or is it as simple as wearing a helmet of some sort?
Williams: TMS in the US is supervised by physicians and it is something that ultimately the physician is responsible for. It is not a particularly complex procedure. In certain research studies, college graduates or research assistants can be trained within a few weeks to learn how to do this. This is not something that requires extensive training for a physician to learn how to do. Unlike other approaches, such as surgery, the learning curve for TMS is modest.
Medgadget: How successful has TMS been for treatment of OCD in comparison to the standard approaches?
Williams: For both OCD and depression, our approach is to recruit treatment-resistant patients, as per the FDA’s approval. If you look at something called STAR*D, which is a depression medication algorithm, and look at where the insurance starts to pay for TMS compared to the next drug, it is substantially better at treating depression than the next drug. In those patients, you get a third of them completely remitted (without symptoms) and half of them meeting responder criteria (have a 50% reduction in their symptoms). The next medication is about half as good as that. The responses are quite durable – a lot of folks end up maintaining their response for six months after just a six-week course of treatment.
Medgadget: Do you foresee this becoming a first-line treatment at some point, or is it still a bit too dangerous or uncharacterized for that?
Williams: It is not too dangerous by any means. The reason why people haven’t gone after first-line treatment approval is simply because the placebo response rate in people that are medication-naïve is extraordinarily high. Most people get better with whatever the first-line treatment is. To do a study to get approval as a first-line treatment for depression, you have to recruit thousands of patients in a trial. The cost of doing something like that is very prohibitive.
Medgadget: Do patients have to permanently be on TMS?
Williams: It is not a permanent solution. It is similar to dialysis, in that you have to do it repeatedly. The good news about TMS, unlike dialysis, is that you can do a six-week course and without any other treatment, about 2/3 of people will maintain a response up to the six-month mark. If you give booster TMS courses (not a full-course), you get it closer to 90% of people maintaining their response. Whether it be treatment-resistant depression or treatment-resistant OCD, those folks are not at all bothered by this idea of coming back in – they are just happy that they are feeling better. As it stands now, this is kind of a network normalizer that fades in time and needs to be re-established over the course of the patient’s illness.
Medgadget: How are the specific targets areas of the brain identified?
Williams: For the majority of the targets that have been established, it is the combination of animal studies and anatomy homology, trial and error and lesion studies, which are observations of a condition affecting a certain area and leading to altered behavior. This combination has led us to the current construction of targets, which is very likely an incomplete map. It is very likely that there are types of depressions, OCD, and other conditions that need different targets or where you may need to use multiple targets.
Medgadget: You mentioned addiction targets are also known. Has TMS been used in that context?
Williams: There are a number of folks around the world looking at trying to use TMS for addictions such as alcohol dependence, cocaine, methamphetamines, and heroin. BrainsWay has a coil for smoking cessation that they have ongoing trials for. In some ways, it is a good approach, because I have yet to meet a TMS-addicted individual. It is not possible. The problem that we have with addiction treatment, particularly with opioid treatment, is that you’re just replacing the problem with what we view to be less problematic substances, but they also have addiction potential. In the case of TMS, the beauty is that it’s an entirely different approach trying to change brain network function back to something more physiologically normal.
Medgadget: In those cases, the approach is to use TMS to inhibit addiction centers?
Williams: Some people have used TMS to inhibit certain areas that are hyperactive in people with drug-seeking or addictive behaviors. Others have tried to excite areas that are under-active in cases of addiction, like cognitive control centers.
Medgadget: Could you share some of the success stories that you have observed yourself and how this treatment has impacted patient lives?
Williams: I have both a clinical TMS practice and a lab in which I run experimental TMS protocols for various problems. Generally, between those two populations of patients, it is extraordinarily both rewarding and effective. Patients have regularly reported that this a life-changing intervention for them, putting them back on the path of having a normal life. For an intervention as low risk as this is, something that has only a 1 in 30,000 risk of a short seizure that has never resulted in any long-term problem, it is extraordinarily powerful and effective. I had one lady who was getting treated and was doing fairly well early on treatment, but we found out she was still drinking alcohol, which is a risk for having TMS-related seizures with certain stimulation protocols. We switched her to one that was not risky from a TMS seizure standpoint. Not only does she have improvement with her depression, but she also stopped drinking, went to AA for the first time in her life, and went back to work. We have seen a number of people who have had that kind of success. Yes, I have another patient with pretty severe OCD, and after a couple of days of treatment was able to leave the hospital and do quite well in the world.
Medgadget: Amazing! So, where do you see the clinical use of TMS in 10 or 15 years?
Williams: There is an emerging idea of electroceuticals, a term for the use of neuromodulation as a treatment. I think it is going to scale out as much, or potentially even more than, where drugs are right now. I think TMS plays largely into that group of neuromodulation treatments. As this field of medicine continues to expand and emerge, TMS will, at least for foreseeable future, be a very central part of that.

For more information, check out Dr. Nolan’s Stanford Profile…
